Summary
The ongoing phasedown of hydrofluorocarbons and other high global‑warming‑potential (GWP) refrigerants has profound implications for the U.S. Department of Defense (DoD). Employing low-GWP alternatives requires trade-offs between flammability and volumetric capacity (i.e., component sizing). The flammability aspect of low-GWP refrigerants poses additional challenges for military applications due to safety concerns. This study investigates minimally flammable alternatives to R-134a and R-1234yf by using a holistic approach that encompasses both thermodynamic aspects and flammability characteristics. A thermodynamic screening framework was developed to assess binary, ternary, and quaternary mixtures under varying operating conditions. Various vapor‑compression‑cycle architectures for transport applications were considered. The simulations quantitatively assessed trade-offs among seasonal coefficient of performance, GWP, flammability, and volumetric cooling capacity. Based on these results, recommendations for refrigerant blends meeting military specifications and sustainability goals have been made. Hazards testing under relevant military conditions was used to determine safety and performance. These conditions were simulated in a blast chamber with high-speed cameras at different pressure, temperature, and humidity conditions to better understand the refrigerant’s behavior. Results from this study will provide the DoD with safe and environmentally conscious refrigerant-blend options.
Introduction
Due to the high GWP of R-134a and potential supply-chain issues, new refrigerants and refrigerant blends are being considered as replacements [1]. Many of these have been found to be at least marginally flammable. However, the unique circumstances of military applications have led to a search for a refrigerant with both low GWP and flammability.
The American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE) 34 standard [2] describes the established method of classifying refrigerant flammability. This is done in a glass flask of refrigerant and air with a small spark between electrodes. The refrigerant is classified from A1, with no flame propagation, to A3, for higher flammability, based on heat of combustion and lower flammability limit [3]. For this testing, the temperature, humidity, and pressure are all controlled. While this is helpful for determining refrigerant flammability in ideal conditions, it leaves the question of how will these refrigerants behave in nonideal conditions?
Besides flammability and GWP, the replacement refrigerant’s performance in the system is key to the overall environmental impact. To identify candidate refrigerants, numerical fluid screening based on Bell et al. [1] was used to determine mixtures to replace R‑134a in a military mobile air-conditioning (MAC) system. In these systems, the refrigerant’s flammability is of particular concern due to the operational environment. Once a candidate refrigerant is identified, it can then be tested experimentally using the methods developed in this work to confirm its safety when used in a military MAC system.
The objective of this work is to develop and apply an experiment to find a refrigerant or blend that successfully balances low GWP and low flammability for military MAC systems. To begin answering this question, an experimental setup was designed to create and test nonideal conditions and ignition sources to study how the refrigerants will respond to harsh conditions, such as those potentially seen in military situations.
Experimental Setup for Flammability Study
The flammability experiments were developed and performed in Maurice J. Zucrow Laboratories at Purdue University. The experimental setup mainly consisted of a spherical blast chamber to contain the ignition, as seen in Figure 1a.
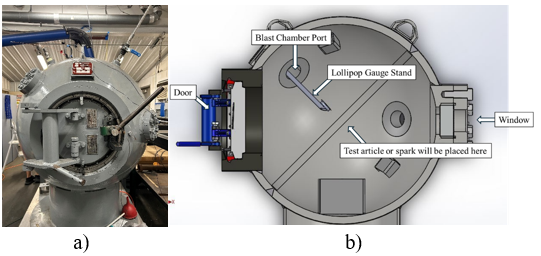
The chamber was airtight and could be filled with the selected refrigerant using a programmable refrigerant pump and scale. The amount of refrigerant used for each test was chosen to be the midpoint between the upper and lower flammability limits for each refrigerant. All photos and videos in this experiment were taken through the window, as shown in Figure 1, using a high‑speed camera (Phantom TMX7510) with a frame rate and exposure time of 300 FPS and 400 µs, respectively, and were adjusted throughout the duration of testing based on anticipated flames.
Three ignition sources were selected: (1) a 4-kV spark, (2) a heated nichrome wire, and (3) a small propellant sample composed of 90-wt% ammonium perchlorate (AP)-hydroxyl terminated polybutadiene (HTPB). These sources were selected because they yielded a high-enough energy to reach or exceed the minimum ignition energy for most refrigerants. Additionally, each source delivered a distinct form of ignition energy, allowing for an analysis of refrigerant behavior under varying energy levels.
High-voltage sparks were selected as an ignition source because they replicated potential electrical accidents, such as the unintentional discharge of a high-voltage power source or a sudden break in electrical wiring. Among the three ignition sources, this method most closely aligned with conventional testing standards. In this series, this high-voltage spark was created using a capacitive discharge unit (Teledyne Risi FS-43) typically used for firing detonators (Figure 2).


Finally, propellant was used as an ignition source because it could introduce different elements as well as a longer burn time (heat pulse) that mimics what would potentially be seen in a military combat scenario. The propellant used was 0.2 g of 90-wt% AP-HTPB (Figure 4).

The process for each test consisted of selecting an ignition source and wiring it into the chamber; selecting a refrigerant, then sealing the chamber; and pumping in the desired amount of refrigerant. After the ignition source was initiated, the refrigerant’s behavior was closely observed. A go or no-go result was determined by identifying a flame that was not present with just the ignition source in air alone. In this test series, a go result indicated that the refrigerant was flammable under the chosen conditions.
Numerical Fluid Screening
Numerical fluid screening in this work follows the method detailed in Bell et al. [1], where 15 different fluids, including hydrofluorocarbons and hydrofluoroolefins, were used to generate binary, ternary, or quaternary mixtures using the National Institute of Standards and Technology Reference Fluid Thermodynamic and Transport Properties Database (REFPROP) version 10 [4]. REFPROP can evaluate a mixture’s properties, including pressure, temperature, and enthalpy, based on the mixture’s composition. This way, a refrigeration cycle can be constructed. Further, a mixture’s performance, including coefficient of performance (COP), volumetric cooling capacity (VCC), and GWP, can be calculated and compared for their operation in the same cycle under the operating condition for a MAC system. A mixture’s COP is essential, as it directly reflects the energy efficiency of the cycle. The VCC is the amount of cooling capacity the cycle can deliver per unit volume. A larger VCC means the MAC system can be smaller in size, which can save space on the vehicle while avoiding the risk of being targeted during combat. Finally, the GWP shows the refrigerant’s impact on environment and its carbon footprint.
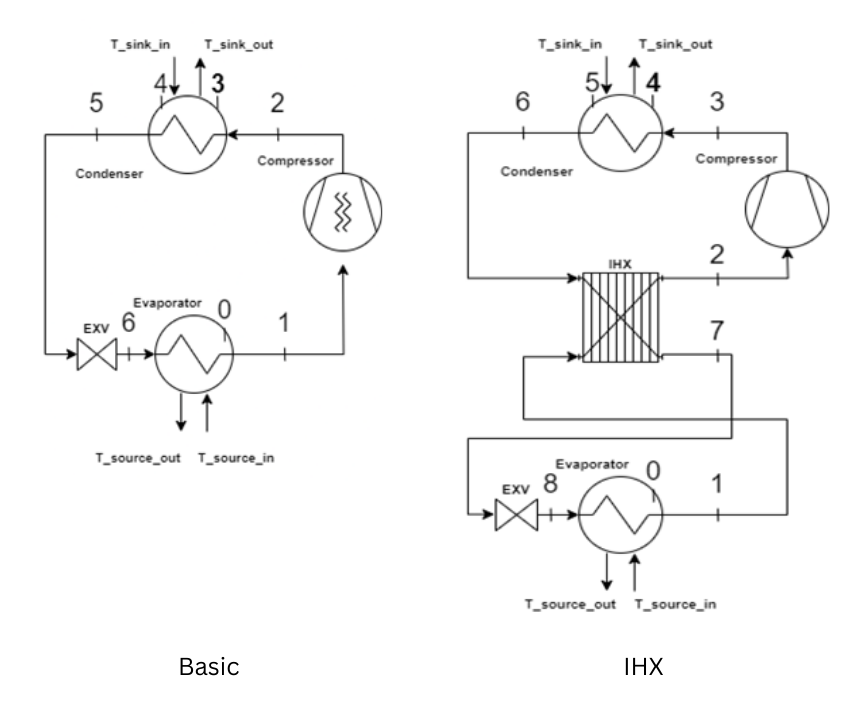
Figure 5 shows the cycle schematics for both a basic four-component cycle and a cycle enhanced with an internal heat exchanger (IHX). The enhanced cycle exchanges heat between the high-pressure liquid refrigerant exiting the condenser and the low-pressure vapor exiting the evaporator. Compared to the basic cycle, the IHX cycle can enhance the MAC system’s performance by increasing the cooling capacity since the liquid exiting the condenser is further subcooled and the refrigerant’s enthalpy at the evaporator inlet is decreased. Additional cycle configurations such as cascade cycles or secondary fluid cycles that can bring additional performance benefits to the MAC system can also be employed in the screening process.
The mixture’s flammability can be calculated using the method detailed in Linteris et al. [3], where a mixture’s adiabatic flame temperature (Tad) and its ratio of the number of fluorine atoms to the total number of fluorine plus hydrogen atoms are used to estimate the flammability of the mixture, also referred to as fluorine loading (F/(F+H). Both parameters are obtained from Cantera, an open-source package for solving chemical kinetics, thermodynamics, and transport properties [5]. It should be noted that fluorine loading is calculated using the reactants of the chemical reaction that factors in the humidity in the air. A flammability index Π can be calculated using the following equation:
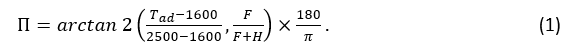
Once a mixture is generated, its performance inside the MAC system and its flammability are calculated. Considering both, a holistic screening process is performed based on a series of selection criteria to screen out the desired mixtures. Once top-performing mixtures are identified, they can be tested in the experimental setup to confirm their flammability to ensure they are safe to use inside a military MAC system.
The results from an example screening can be seen in Figure 6. In this case, ternary mixtures were generated at 0.04-mol fraction steps for 15 different fluids to identify midterm mixtures to replace R-134a in an IHX cycle operating at 40 °C ambient temperature and 20 °C interior temperature. As seen in Figure 6, the following screening criteria were applied: GWP100 yr < 500 for midterm solutions, COP and VCC of at least half of that for R-134a system, and flammability index Π < 36.5. It should be mentioned that the flammability index separating A1 (nonflammable) and A2L (mildly flammable) fluids is Π = 36. In this case, the flammability limit was set slightly higher to allow mixtures close to the flammability limit to still be considered to account for uncertainty in flammability predictions.
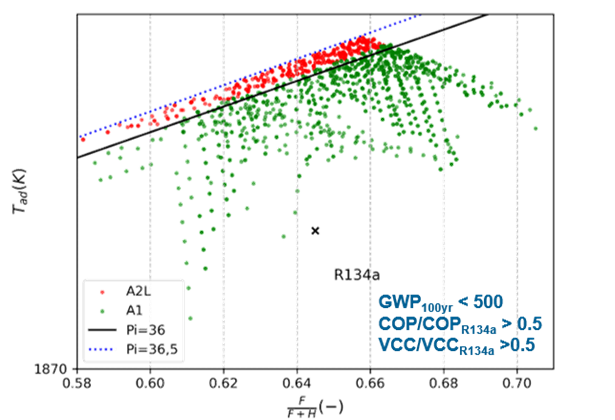
Among the selected mixtures, the one with the highest COP is R‑227ea(0.08)/R‑1234ze(E)(0.88)/R-1336mzz(E)(0.04). The COP for this fluid is 4.60, which is 1.99% higher than that of the baseline R-134a. Its VCC is 2.27, which is 7.79% lower than that of the baseline refrigerant. The GWP of this mixture is 379.9, which makes it a good choice for replacing R-134a as a midterm solution. The mixture’s ASHRAE flammability class is A2L, but its flammability index is lower than 36.5. This mixture can then be tested in the experimental setup presented in this article to test its flammability to confirm if it can be safely used in a military MAC system.
Results of Preliminary Flammability Experiments
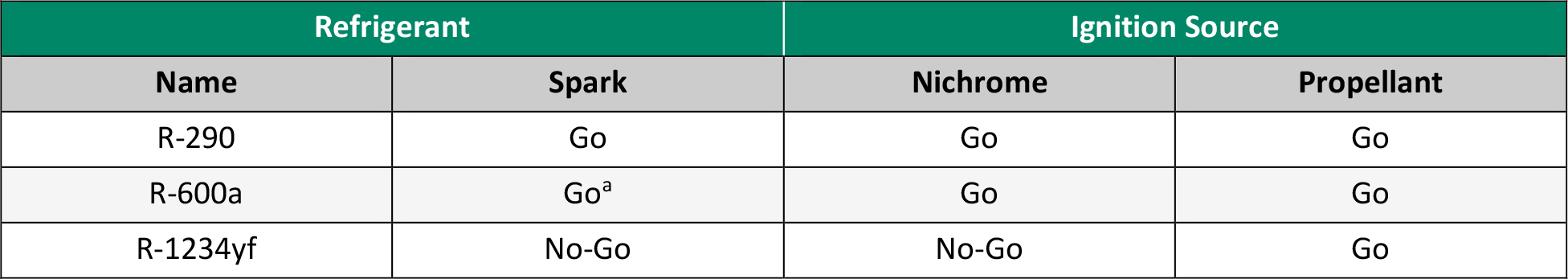
Before the mixtures from the screening could be tested in the experimental setup, some sample refrigerants were tested first in a preliminary study to validate the method. The refrigerants selected for this preliminary study were R-290, R-600a, and R-1234yf. These were selected due to their range in flammability classifications. R-290 and R-600a were highly flammable and helped visualize what ignition would look like under each condition, while R‑1234yf was much less flammable and allowed for determining if the higher energy sources were enough to ignite the refrigerant. Table 1 details the properties of selected refrigerants, and Table 2 shows the amounts used for each test.
Table 1. Properties of Selected Refrigerants (Source: C. E. O’Malley)
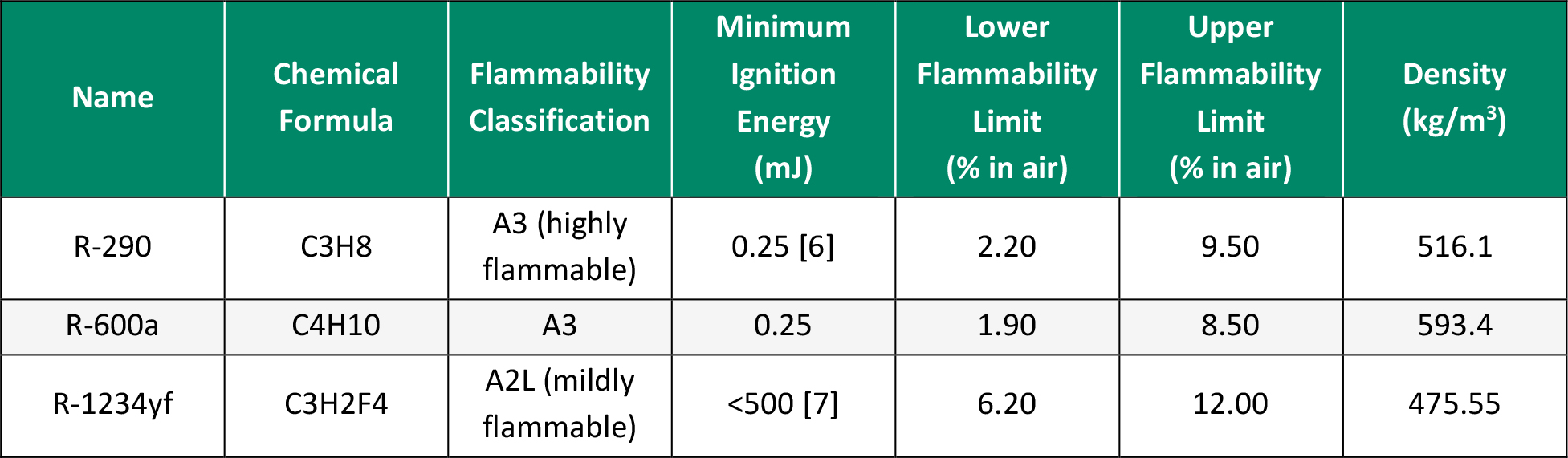
Table 2. Amount of Refrigerant Used in Each Test (Source: C. E. O’Malley)
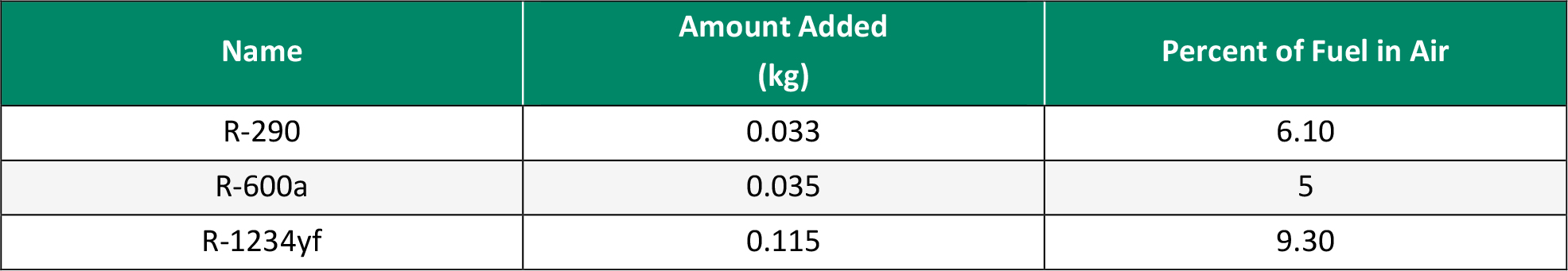
Table 3. Results of Ignition Source Testing on Refrigerants (Source: C. E. O’Malley)
The refrigerants tested with each of the ignition sources are shown in Figures 7–9. R-290 is classified as very flammable, so the behavior shown in the images is expected (Figure 7). It is observed that the flame propagates quickly and dramatically, indicating that in a disaster scenario, R-290 would not be a good refrigerant to have in a system, as it combusts quite easily. R-600a is another refrigerant classified as very flammable, so the large flames seen in the third panel of these images were expected (Figure 8).
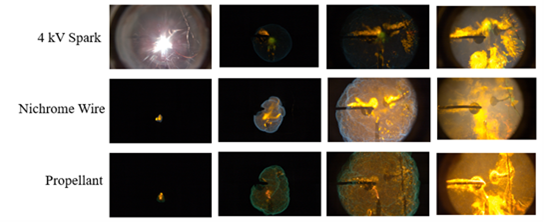


R-1234yf is classified as minimally flammable, so it was expected that some of the ignition sources would not produce flame (Figure 9). The appearance of the spark and the nichrome‑wire ignitions appeared almost exactly as it did in the tests with no refrigerant added. The only ignition source that was affected by the R-1234yf was the propellant. The flame appeared brighter and larger than in control tests (without refrigerant), indicating that some of the refrigerant was either also burning or adding species to influence the burning of the propellant. This indicates that in a military style scenario, R-1234yf would not likely contribute to an electrical or hot-plate ignition, but, if propellants were introduced, the refrigerant would potentially exacerbate the situation.
This test series concluded that the ignited refrigerants behaved predictably, even when exposed to higher-level energy sources. However, in some cases, different responses to various ignition sources could be seen, such as R-600a displaying different behavior when exposed to spark ignition.
These experimental results show the proposed flammability testing method can be used to experimentally determine the flammability of gases at different ignition conditions. It was seen in experimental testing that the class A3 refrigerants remained flammable across all selected ignition sources, while the A2L refrigerant only ignited with the propellant. For future studies, this indicates that ignition sources will have an impact on flammability. This process will next be applied to the blends proposed by the numerical screening methods previously described.
Conclusions
The flame behavior seen in these tests proved that refrigerants would ignite somewhat predictably, even when exposed to simulated military-level threats, depending on flammability. However, in some cases, the ignition method played a role. Further testing will focus on higher‑energy ignition sources and testing blends of refrigerants. One of these blends will be the one with the highest COP—R-227ea(0.08)/R-1234ze(E)(0.88)/R-1336mzz(E)(0.04). Because this blend performed the best, as predicted by the modeling code, its behavior under physical flammability testing will determine its suitability for use in military systems.
Acknowledgments
This work was funded by the U.S. Department of Defense and the Strategic Environmental Research and Development Program under the proposal WPSON-23-3793 “Holistic Optimization of Air-Conditioning Systems for Military Use With Low-GWP Refrigerants.”
References
- Bell, I. H., P. A. Domanski, M. O. McLinden, and G. T. Linteris. “The Hunt for Nonflammable Refrigerant Blends to Replace R-134a.” International Journal of Refrigeration, pp. 484–495, doi: 1016/j.ijrefrig.2019.05.035, 2019.
- American Society of Heating, Refrigerating, and Air-Conditioning Engineers. “Designation and Safety Classification of Refrigerants.” ANSI/ASHRAE Standard 34-2022, 2022.
- Linteris, G. T., I. H. Bell, and M. O. McLinden. “An Empirical Model for Refrigerant Flammability Based on Molecular Structure and Thermodynamics.” International Journal of Refrigeration, vol. 104, pp. 144–150, doi: 1016/j.ijrefrig.2019.05.006, 2019.
- Huber, M. L., E. W. Lemmon, I. H. Bell, and M. O. McLinden. “The NIST REFPROP Database for Highly Accurate Properties of Industrially Important Fluids.” Industrial & Engineering Chemistry Research, vol. 61, no. 4, doi: 1021/acs.iecr.2c01427, 21 June 2022.
- Goodwin, D. G., H. K. Moffat, and R. L. “Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes.” Version 2.3.0, http://www.cantera.org, 2017.
- Ning, Q., G. He, W. Sun, M. Fan, X. Li, and Z. Hong. “R290 Leakage Hazards Assessment of a 1 HP Split-Type Household Air Conditioner by Concentration Detection and Ignition Experiment.” International Journal of Refrigeration, vol. 139, pp. 70–83, doi: http://dx.doi.org/10.1016/j.ijrefrig.2022.04.005, 2022.
- Sadaghiani, M., A. Arami-Niya, D. Zhang, T. Tsuji, Y. Tanaka, Y. Seiki, and E. F. May. “Minimum Ignition Energies and Laminar Burning Velocities of Ammonia, HFO-1234yf, HFC‑32 and Their Mixtures With Carbon Dioxide, HFC-125 and HFC-134a.” Journal of Hazardous Matter, vol. 407, doi: 1016/j.jhazmat.2020.124781, 2021.
Biographies
Claire E. O’Malley is a graduate student in mechanical engineering at Purdue University and a graduate student intern at Sandia National Laboratories. Her research focuses on refrigerant flammability and safety for use in military applications. She has presented at several national conferences. Ms. O’Malley holds a bachelor’s degree from the University of New Mexico and is working on her master’s thesis.
Changkuan Liang is a research scientist with the School of Mechanical Engineering at Purdue University, currently working at Purdue’s Ray W. Herrick Laboratories in thermal systems, heating, ventilation, and air conditioning. His research interests include low global‑warming potential (GWP) and flammable refrigerant selection and application, compressor development for membrane applications, domestic refrigerator/freezer development, and refrigerant mixture screening for next-generation low-GWP alternatives for military mobile air-conditioning applications. He has authored or co-authored numerous peer-reviewed publications. Dr. Liang holds a Ph.D. in mechanical engineering from Purdue University.
Enrique A. Velazquez is a mechanical test engineer with X-Bow systems, performing solid rocket motor static fire testing. His research interests include combustion and energetic materials. Mr. Velazquez holds a master’s degree in mechanical engineering from Purdue University.
Robert E. Ferguson is a research scientist at the School of Mechanical Engineering at Purdue University, currently working at Purdue’s Maurice J. Zucrow Laboratories in combustion and energetic materials. His research interests include solid propellants, fuels, explosives, resource utilization, fire suppression of lithium-ion batteries in thermal runaway, and detonation velocity using machine-learning techniques. He has authored or co-authored numerous peer-reviewed publications. Dr. Ferguson holds a Ph.D. in mechanical engineering from the University of Texas at El Paso.
Listier A. Otieno is a second-year master’s student and research assistant in the School of Mechanical Engineering at Purdue University, working at Herrick Labs. His work focuses on modeling and experimental investigation of heat-pump systems for mobile air‑conditioning applications. His research interests include low-GWP refrigerants, cycle optimization for compact and high-efficiency thermal systems, advanced vapor compression technologies, design and construction of an experimental test stand to evaluate alternative refrigerants, and cycle configurations under real‑world automotive conditions.
Steven F. Son is the Alfred J. McAllister professor of mechanical engineering at Purdue University and is affiliated with Purdue’s Maurice J. Zucrow Laboratories. His research focuses on combustion, with an emphasis on energetic materials, including nanoscale energetic materials, microscale energetics (micro-energetics), heterogeneous combustion, reactive materials, combustion synthesis, and explosives safety. He has presented at many national and international scientific meetings and authored over 300 scientific publications. He is a fellow of the American Society of Mechanical Engineers and the Combustion Institute and an associate fellow of the American Institute of Aeronautics and Astronautics. Dr. Son holds a Ph.D. from the University of Illinois at Champaign-Urbana.
Davide Ziviani is an associate professor of mechanical engineering at Purdue University and the codirector of the Center for High Performance Buildings at the Ray W. Herrick Laboratories. His research focuses on advanced heat-pump and thermal-management systems for different applications, including buildings, space habitats, data centers, and military applications. He is actively involved with the American Society of Heating, Refrigerating, and Air-Conditioning Engineers; the International Institute of Refrigeration; Commission B2 Refrigerating Equipment; and the Purdue Military Programs Committee. He has authored or co-authored more than 150 journal articles and conference papers. Dr. Ziviani holds a B.S. and M.S. from the University of Ferrara in Italy and a Ph.D. from Ghent University in Belgium.


